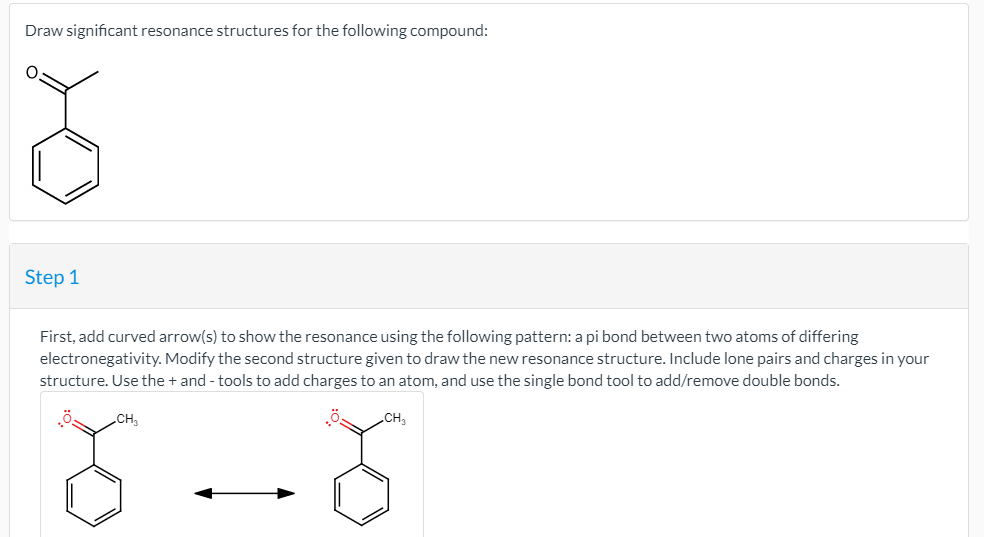
Draw Significant Resonance Structures For The Following Compound
Draw Significant Resonance Structures For The Following Compound - You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web video answer:in this problem were given this molecule here and we are asked to draw significant resonance structures so remember this is where we see a shift of electrons and our con activity is the same, but you may see pi bonds and 1 electron pairs move. They are used when there is more than one way to place double bonds and lone pairs on atoms. Step 1 x your answer is incorrect. Indicate which would be the major contributor to the resonance hybrid. Include lone pairs and charges in your structure. It is the one with the least formal charges that adds up to zero or to the molecule’s. So, first remember that our oxygen has 2 lone pairs on it. Circle the most significant contributor in each case. Include lone pairs and charges in your structure. Step 1 x your answer is incorrect. Determine which resonance patterns are present, and identify all of the significant resonance structures for the given compound. Include lone pairs and charges in your structure. A molecule or ion with such delocalized electrons is represented by several resonance structures. Web concept explainers question draw significant resonance structures for the following compound: O step 1 your answer is incorrect first, add curved arrow (s) to show the resonance using the following patterns a pi bond between two atoms of differing electronegativity, modify the second structure given to draw the new resonance structure. Web draw significant resonance structures for the following compound: Web practice the skill 02.260 for the given compound, draw all. Step 1 x your answer is incorrect. Expert solution trending now this is a popular solution! Use the concept of resonance to explain structural features of molecules and ions. The total of valence electrons is 16. Care octant ho estradiol (female sex hormone) testosterone (male sex hormone) this problem has been solved! First, add curved arrow (s) to show the resonance using the following pattern: Indicate which would be the major contributor to the resonance hybrid. Care octant ho estradiol (female sex hormone) testosterone (male sex hormone) this problem has been solved! Circle the most significant contributor in each case. Web draw significant resonance structures for the following compound: Use the concept of resonance to explain structural features of molecules and ions. Web video answer:in this problem were given this molecule here and we are asked to draw significant resonance structures so remember this is where we see a shift of electrons and our con activity is the same, but you may see pi bonds and 1 electron pairs. Determine the relative stability of resonance structures using a set of rules. First, add curved arrow (s) to show the resonance using the following pattern: Web draw significant resonance structures for the following compound: Step 1 x your answer is incorrect. It is the one with the least formal charges that adds up to zero or to the molecule’s. A resonance form is another way of drawing a lewis dot structure for a given compound. First, add curved arrow (s) to show the resonance using the following pattern: Web draw significant resonance structures for the following compound: Draw all significant resonance structures for the compounds illustrated below. Web video answer:in this problem were given this molecule here and we. Draw all significant resonance structures for the compounds illustrated below. 2.49 write a condensed structural formula for each of the following compounds: First, add curved arrow (s) to show the resonance using the following pattern: Answer please provide the structure or the name of the compound, and i will be happy to help you draw its significant resonance structures. Find. You must show clearly drawn arrows that connect each resonance structure. Indicate which would be the major contributor to the resonance hybrid. Web resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single lewis formula. (20pts) draw all reasonable resonance structures for the following compound. Modify the. Web chemistry chemistry questions and answers 2.41 draw significant resonance structures for the following compound this problem has been solved! Step 1 get help answering molecular dray collapse x your answer is incorrect. They are used when there is more than one way to place double bonds and lone pairs on atoms. Web 1) for the following resonance structures please. Web draw significant resonance structures for the following compound: Indicate which would be the major contributor to the resonance hybrid. 2) draw four additional resonance contributors for the molecule below. Determine the relative stability of resonance structures using a set of rules. Modify the second structure given to draw the new resonance structure. A molecule or ion with such delocalized electrons is represented by several resonance structures. Equivalent lewis structures are called resonance forms. Include lone pairs and charges in your structure. Web draw the resonance structures of molecules or ions that exhibit delocalization. They are used when there is more than one way to place double bonds and lone pairs on atoms. Web video answer:in this problem were given this molecule here and we are asked to draw significant resonance structures so remember this is where we see a shift of electrons and our con activity is the same, but you may see pi bonds and 1 electron pairs move. Web 2.48 draw all significant resonance structures for each of the following compounds: Web concept explainers question draw significant resonance structures for the following compound: Draw significant resonance structures for the following compound: Use the concept of resonance to explain structural features of molecules and ions. (20pts) draw all reasonable resonance structures for the following compound.
draw significant resonance structures for the following compound
Solved Draw significant resonance structures for the
Solved Draw ALL significant resonance structures for the

Solved Draw significant resonance structures for the

Solved Question 73 Draw significant resonance structures for

Draw the most important resonance structures for the following

Draw All Significant Resonance Structures For Each...

Draw Significant Resonance Structures for the Following Compound
Solved Draw a valid resonance structure for the following

SOLVED4) (10 POINTS TOTAL) Draw all significant resonance structures
Include Lone Pairs And Charges In Your Structure.
Web Draw Significant Resonance Structures For The Following Compound:
Step 1 X Your Answer Is Incorrect.
Web Draw The Resonance Structures Of Molecules Or Ions That Exhibit Delocalization.
Related Post: