Draw An Atom Of Oxygen
Draw An Atom Of Oxygen - The oxygen atom will be the central atom; Let’s draw and understand this lewis dot structure step by step. There, we see that the atomic number of oxygen is eight. In the o 2 lewis structure, there is a double bond between the two oxygen atoms, and on each oxygen atom, there are two lone pairs. And both the oxygen atoms have two lone pairs on it. It is four for one o2 molecule; Add up to two electrons to the first electron shell. We can also assume that it has 8 electrons. (generally, the least electronegative element should be placed in the center.) connect each atom to the central atom with a single bond (one electron pair). Web steps of drawing lewis diagram. Look for the total number of bonds forming: It is four for one o2 molecule; Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom. The bohr model of oxygen is drawn with only two electron shells, the first shell contains. Let’s draw and understand this lewis dot structure step by step. It has symbol o and atomic number 8. O 2 ( oxygen) has two oxygen atoms. Web oxygen is the eighth element with a total of 8 electrons. So, oxygen has eight positive particles plus eight negative particles. From this, it can be understood that the geometrical structure of a single h2o molecule is bent. This means that every atom or ion of oxygen has eight protons in its nucleus. Let’s draw and understand this lewis dot structure step by step. Oxygen atom van der waals radius is 152 pm. How does this diagram account for the paramagnetism of o 2? Then place one dot at each side of the symbol. Add up to two electrons to the first electron shell. This means that every atom or ion of oxygen has eight protons in its nucleus. It is explained with the help of. Web lewis structure of o2 (oxygen) contains one double bond between both the oxygen (o) atoms. Let’s draw and understand this lewis dot structure step by step. In a water molecule, an oxygen atom forms two bonds, one to each hydrogen atom. This is the first electron shell. This means that every atom or ion of oxygen has eight protons in its nucleus. Look for the total number of bonds forming: Take a pen and paper with you and try to draw this lewis structure along with. Then place one dot at each side of the symbol. Draw the symbol for oxygen. Here are electron shell atom diagrams for the elements, ordered by increasing atomic number. Web lewis structure of o2 (oxygen) contains one double bond between both the oxygen (o) atoms. How does this diagram account for the paramagnetism of o 2? It is two for each oxygen atom; In the form of shells in the form of orbitals let’s talk about orbitals first. Oxygen atom van der waals radius is 152 pm. Here are electron shell atom diagrams for the elements, ordered by increasing atomic number. From this, it can be understood that the geometrical structure of a single h2o molecule. It is two for each oxygen atom; Oxygen is in group 16/via, so it has six valence electrons. Web oxygen is a chemical element; Here are electron shell atom diagrams for the elements, ordered by increasing atomic number. There, we see that the atomic number of oxygen is eight. Draw another circle around the first shell. Web oxygen is a chemical element; Two fluorine atoms can form a molecule of f 2 in the same fashion. It is explained with the help of. Add up to two electrons to the first electron shell. In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital. Web updated on november 05, 2019. Then place one dot at each side of the symbol. How does this diagram account for the paramagnetism of o 2? In the o 2 lewis structure, there is a double bond between the two oxygen atoms,. Double covalent bonds are forming in an o2 molecule; So, oxygen has eight positive particles plus eight negative particles. Oxygen atom van der waals radius is 152 pm. The best way to represent the electrons is to draw them as two pairs, and two more single electrons. Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom. Web lewis structure of o2 (oxygen) contains one double bond between both the oxygen (o) atoms. Take a pen and paper with you and try to draw this lewis structure along with me. And, it can be shown in two ways: Two fluorine atoms can form a molecule of f 2 in the same fashion. Web oxygen is the eighth element with a total of 8 electrons. It is four for one o2 molecule; The remaining four electrons will go in the 2p orbital. Atoms can form more than one bond. (generally, the least electronegative element should be placed in the center.) connect each atom to the central atom with a single bond (one electron pair). How does this diagram account for the paramagnetism of o 2? Both the atoms will be central;
What is the Electron Configuration of Oxygen Archives Dynamic

Oxygen Valence Electrons (O) Oxygen Valency & Electron Configuration

Drawing Atoms Montessori Muddle

Oxygen Bohr Model (Diagram, Steps To Draw) Techiescientist

Atomic Structure for Oxygen (O2) Best Guide (With Diagrams)

Bohr Model Drawing Of Oxygen at Explore collection

Oxygen Atom Science Notes and Projects

Diagram representation element oxygen Royalty Free Vector
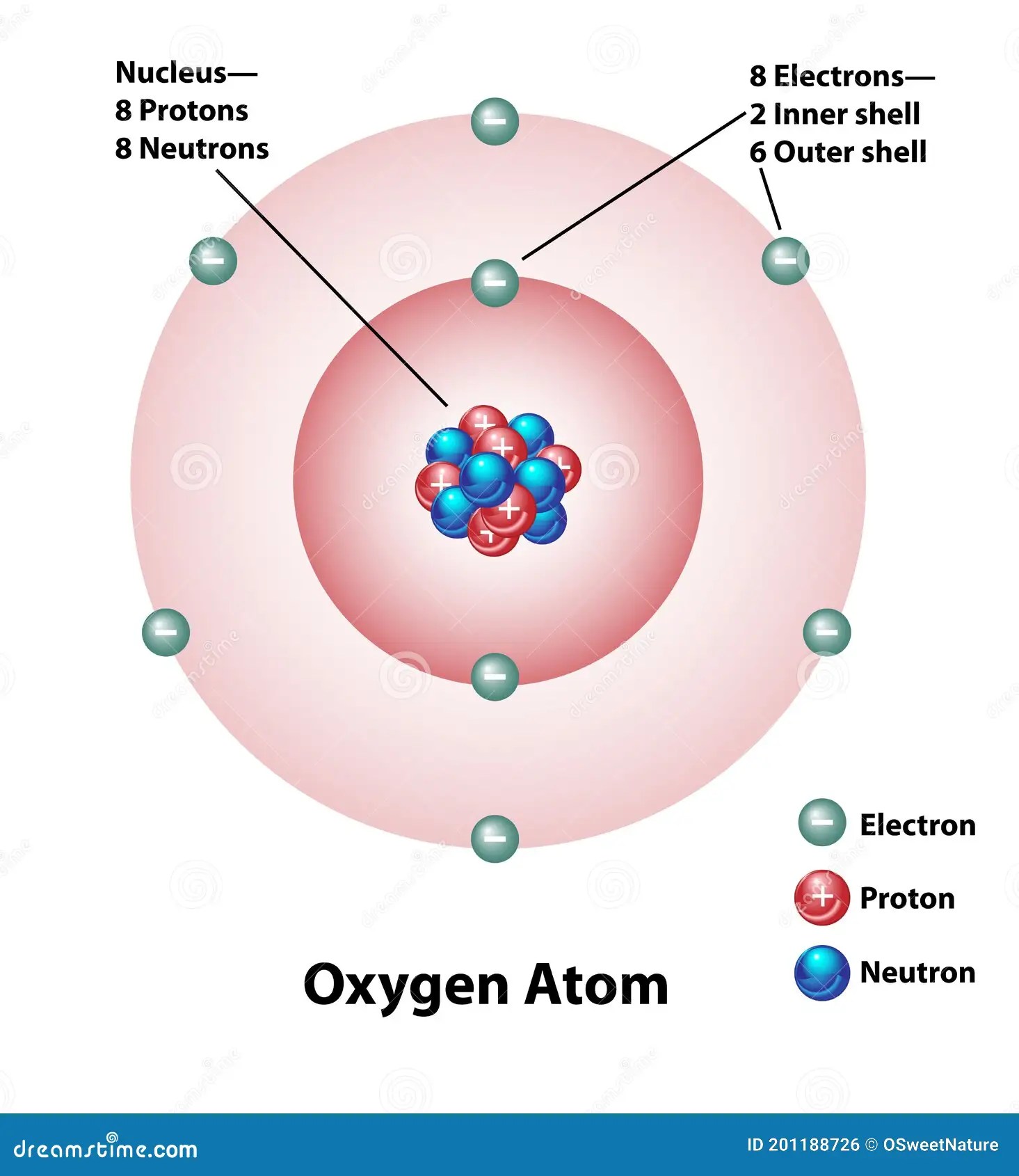
Molecular Structure of an Oxygen Atom Stock Vector Illustration of

Forms of Energy ND Studies Energy Level 2
Web 8 Protons 8 Electrons 8 Neutrons To Know More About The Atomic Structure Of Oxygen, You Need To Learn About The Electronic Configuration.
The Electron Dot Diagram For An Element Shows The Valence Electrons For The Element.
It Is Explained With The Help Of.
2 Electrons Can Go In The First Shell, 8 In The Second, 8 In The Third, And So On.
Related Post: